In vitro compound efficacy
MIC
Minimum inhibitory concentrations (MICs) are defined as the lowest concentration of an antimicrobial that inhibits the visible growth of a microorganism after overnight incubation. MICs are used to determine the in vitro activity of new antimicrobials.
MBC
Minimum bactericidal concentrations (MBCs) are defined as the lowest concentration of antimicrobial that prevents the growth of an organism after subculture on antibiotic-free media.
TKC
Time-kill curves (TKCs) monitor bacterial growth and death over a wide range of antimicrobial concentrations over time.
ANTIBIOGRAM
Based on guidelines produced by the European Committee on Antimicrobial Susceptibility Testing (EUCAST), antibiogram is a report that shows how susceptible strains of pathogens are to a variety of antibiotics.
Biofilm formation inhibition
Biofilms are an ensemble of microbial cells irreversibly associated with a surface. Biofilm plays crucial roles in a variety of infections. Antimicrobial candidate with putative antibiofilm properties can be analyzed on biotic and abiotic surfaces.
Extracellular vesicles
Extracellular vesicles released by bacteria represent a universal, evolutionarily conserved mechanism for intercellular communication, notably through the delivery of virulence factors or modulation of host immunity. Vibiosphen owns the know-how to produce, purify and label extracellular vesicles from Gram negative and Gram positive bacteria.
Compound safety
- Hemolytic test
- Cytotoxicity
- NO/H202 assessment
- MTD maximum tolerated dose
For the development of a new compound, the determination of the therapeutic window, i.e. the threshold between active dose and toxic dose, allows moving forward to clinical phase.
- PK/PD, absorption, distribution, metabolism, and elimination (ADME)
For the development of new compound, pharmacokinetics analyses estimate the fate of the compound from its administration to its elimination by the organism.
- Clinical symptoms (survival, body weight)
- Bacterial loads (CFU, qPCR)
- Inflammation
- Immune cell quantification (ABCvet, Cytometry)
- Histopathological analysis
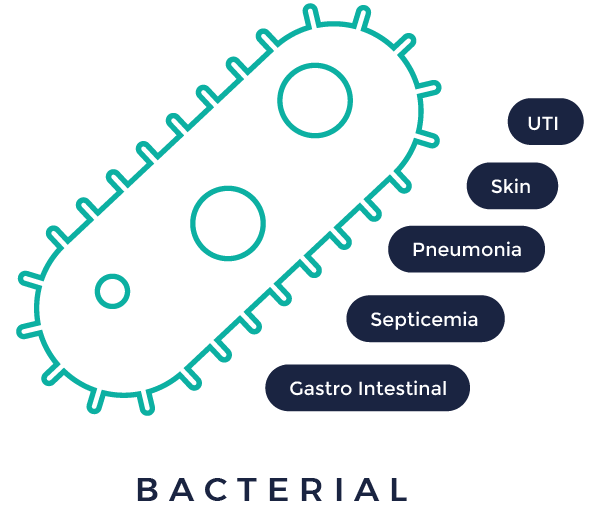
In vivo bacterial infectious diseases
The increasingly relevant problem of nosocomial infection and antibiotic resistance remains a daunting and highly critical unmet medical need with potentially severe and infectious health consequences, as both R&D and federal approval of antibiotics are on the decline. As a biotechnology company, Vibiosphen proposes research into potential future therapies (new antibiotics, peptides, biologics, immune modulator, Phages, combination, ..) against multi-resistant bacteria to treat pneumonia, septicemia, UTI, Gastro-intestinal and skin infections.
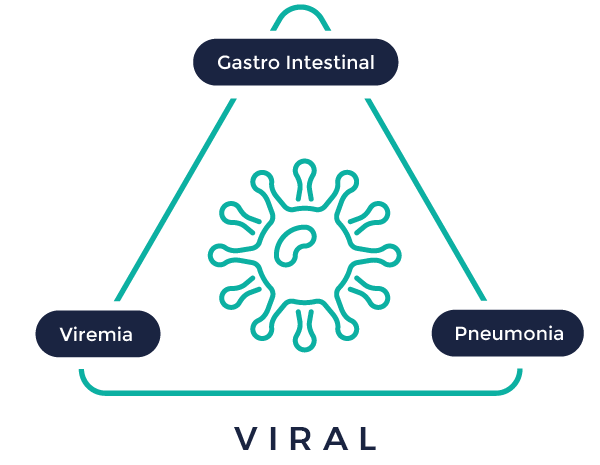
In vivo viral infectious diseases
Viral infections are a small infectious organism. The virus will attach to the surface of the cell and releases its viral DNA or RNA inside the cells which are then replicate by themselves inside the cell to make the cell more infectious and spread from one cell to other leads to viral infection.
Vibiosphen has a high level of expertise in producing predictive pharmacological models and anticipates the requirements for the development of a new molecules in lung and Gastrointestinal infections and viremia.
- Clinical symptoms (survival, body weight)
- Viral loads (TCID50, PFU, qPCR)
- Inflammation
- Immune cell quantification in targeted tissue (Cytometry)
- Histopathological analysis
- Clinical symptoms (survival, body weight)
- Bacterial loads (CFU, qPCR)
- Inflammation
- Immune cell quantification (ABCvet, Cytometry)
- Histopathological analysis
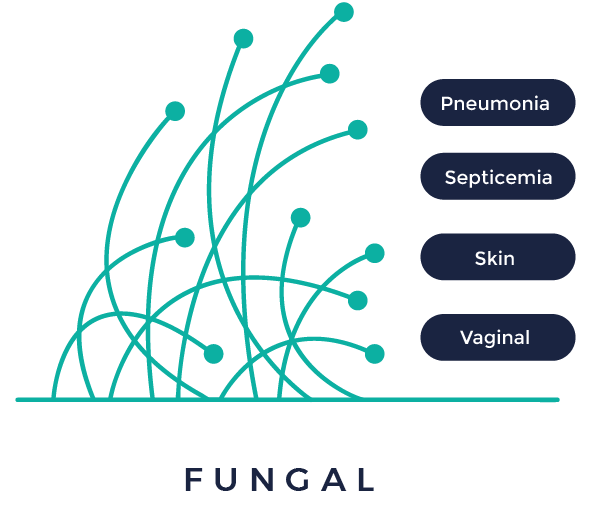
In vivo fungal infectious diseases
Fungal infections are common throughout much of the natural world. In humans, fungal infections occur when an invading fungus takes over an area of the body and is too much for the immune system to handle. It is most commonly seen in immuno-compromised patients with severe neutropenia, oncology patients, or in patients with intravenous catheters.
Our preclinical mouse model has been optimized to assess drug efficacy in pneumonia septicemia, skin and vaginal mouse infectious Model.
Lung organoïds
The organoid technology offers easy handling and cost-effective tools as pre-clinical models but, yet, how organoids are positioned between the already established in vitro and in vivo pre-clinical models has not been properly evaluated, especially in the context of infectious disease. New organoid models for nosocomial bacteria and emergent virus infections, and second at evaluating infected organoids is a relevant information for drug testing.

Immunity and inflammation
- Mode of action (quantification of host biomarkers)
- Quantification of inflammation markers (ELISA, qPCR)
- Cytometry
- Histology
- Serum analysis for the presence of specific antibodies
- Custom ELISA development
- ELISA validation (specifications required by the French Haute Autorité de Santé)
